MATH+
Hospital Treatment Protocol for COVID-19
The following is our recommended approach to COVID-19 in the hospitalized patient, based on the best (and most recent) literature. It is provided as guidance to healthcare providers worldwide and should only be used by medical professionals in formulating their approach to COVID-19. Patients should always consult with their provider before starting any medical treatment. As this is a highly dynamic topic, we will update these guidelines as new information emerges. Please ensure you are using the latest version of this protocol.
The core principle of MATH+ is the use of anti-inflammatory agents to dampen the “cytokine storms,” together with anticoagulation to limit the microvascular and macrovascular clotting, and supplemental oxygen to help overcome the hypoxia.
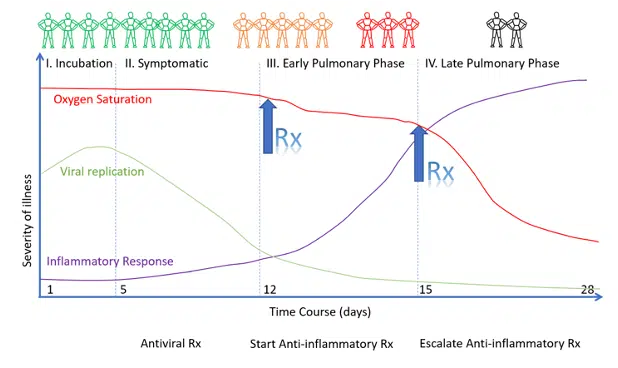
It is critically important to recognize that infection with SARS-CoV-2, the virus that causes COVID-19, progresses through stages. Treatment approaches are therefore highly stage-specific. Antiviral therapy is likely to be effective only during the viral replicative phase. Anti-inflammatory therapy is expected to be effective during the pulmonary phase and possibly the post-COVID phase.
The pulmonary phase of COVID-19 is a treatable disease; it is inappropriate to limit therapy to “supportive care” alone. As patients progress down the pulmonary cascade the disease becomes more difficult to reverse. The implications of this are two-fold:
- Early treatment of the pulmonary phase is ESSENTIAL to a good outcome.
- Treatment in the late pulmonary phase may require escalation of the dose of corticosteroids as well as the use of salvage methods (i.e., plasma exchange). However, patients who present in the late pulmonary phase may have progressed to the irreversible pulmonary fibroproliferative phase.
The information in this document is our recommended approach to treating the hospitalized COVID-19 patient, based on the best (and most recent) literature.
It is provided as guidance to healthcare providers worldwide on the early treatment of COVID-19. Patients should always consult with their provider before starting any medical treatment.
New medications may be added and/or changes made to doses of existing medications as further evidence emerges. Please check this page frequently to be sure you are using the latest version of this protocol.
A note about anesthesia and surgery:
Please notify your anesthesia team if you are using the following medications and/or nutraceuticals as they can increase the risk of Serotonin Syndrome — a life-threatening condition — when opioids are administered:
- Methylene blue
- Curcumin
- Nigella Sativa
- Selective Serotonin Reuptake Inhibitors (SSRIs)
For more information on nutritional therapeutics and how they can help with COVID-19, visit our guide to Nutritional Therapeutics. For additional information, the rationale behind these guidelines, and other optional treatments, see ‘A Guide to Managing the Hospitalized COVID-19 Patient’.
Early treatment is critical and the most important factor in managing this disease.
Overview of MATH+ and Key Concepts
The MATH+ protocol was created to treat patients in hospital and intensive care units, based on using therapies such as anti-inflammatory corticosteroids (Methylprednisolone), high-dose intravenous Vitamin C (Ascorbic acid), Vitamin B1 (Thiamine), and an anticoagulant (Heparin), plus co-interventions such as antivirals and supplements (MATH+).
The protocol was developed in late 2020, at a time when nearly all public health agencies and health care societies were recommending “supportive care only,” based on an incorrect assumption that COVID-19 represented a viral pneumonia and no anti-coronaviral therapy existed.
The core principle of MATH+ is the use of anti-inflammatory agents to dampen the “cytokine storms,” together with anticoagulation to limit the microvascular and macrovascular clotting, and supplemental oxygen to help overcome the hypoxia. Endothelial damage and an imbalance of both innate and adaptive immune responses, with aberrant macrophage activation, plays a central role in the pathogenesis of the severe COVID-19 disease.
COVID is an extraordinarily complex, yet treatable, disease; many of its mysteries are still unravelling. However, a few concepts are key to its management:
Figure 1. The Course of COVID-19 and General Approach to Treatment
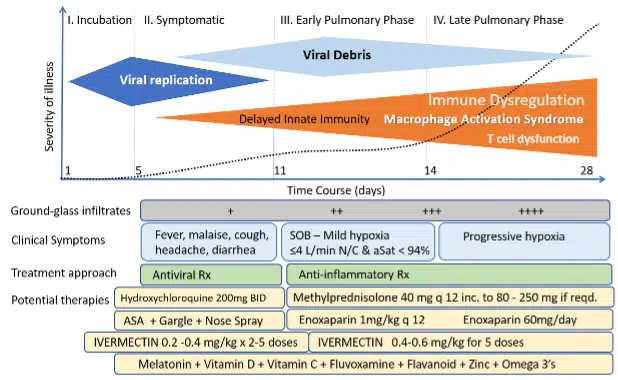
Figure 2. Timing of the Initiation of Anti-Inflammatory Therapy
This protocol is solely for educational purposes regarding potentially beneficial therapies for COVID-19. Never disregard professional medical advice because of something you have read on our website and releases. This protocol is not intended to be a substitute for professional medical advice, diagnosis, or treatment with regard to any patient. Treatment for an individual patient should rely on the judgement of a physician or other qualified health provider. Always seek their advice with any questions you may have regarding your health or medical condition. Please note our full disclaimer at: www.flccc.net/disclaimer

